release date:2021-11-29

Respond to the development trend of the COVID-19 epidemic, WHO, CDC and other international organizations are constantly updating the operational guidelines for the prevention and control of neocrown. Several countries internationally (USA, many EU countries, Japan, etc.) recognize the diagnostic value of antigen in the early stage of neo-crown infection. In Europe, rapid antigen testing plays an important role in the auxiliary screening of suspected patients, screening of asymptomatic high-risk groups and regular surveillance with shorter testing time and lower cost, especially in the case of limited RT-PCR testing capacity.
SARS-COV-2 Antigen Test(Latex immunochromatography method)
Technical principle: latex immunochromatographic technique, monoclonal antibody double antibody sandwich method.
Test sample: Human nasal secretion and oropharyngeal secretion samples.
Detection target: Novel coronavirus (SARS-CoV-2) N antigen in the sample.
Scope of application: Generally used for testing samples during the acute infection period, i.e. within 7 days of the onset of symptoms in the suspected population.

SARS-COV-2 and Influenza A/B Antigen Combo Test(Latex immunochromatography method)
Technical principle: Latex immunochromatographic technique, monoclonal antibody double antibody sandwich method.
Samples: Human nasal secretion, oropharyngeal secretion samples.
Test target: Novel coronavirus (SARS-CoV-2) N antigen, influenza A virus antigen and influenza B virus antigen in the sample.
Scope of application: Generally used for testing samples during the acute infection period, i.e. within 7 days of the onset of symptoms in the suspected population.
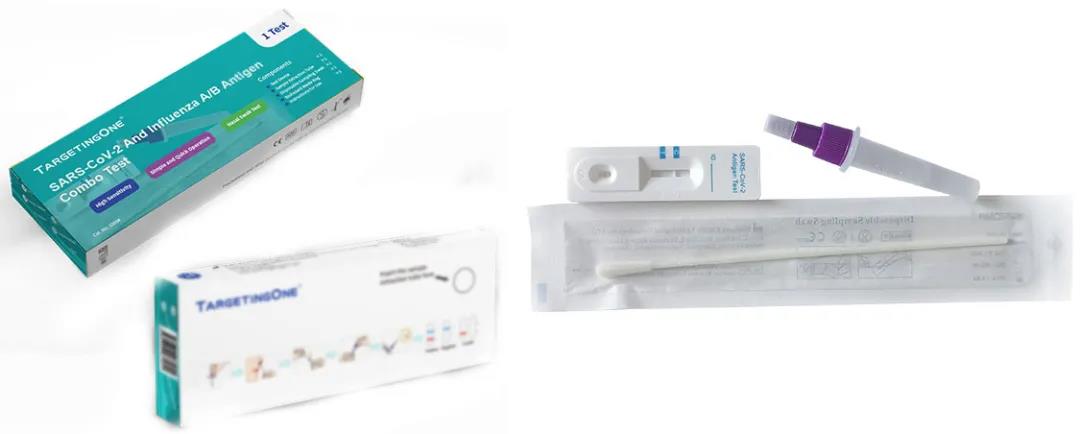
Product advantages
High specificity: detection of national negative/positive reference products, the compliance rate are 100%.
High sensitivity: The Limit of Detection(LOD) detection is 100 TCID50 /mL, reaching the leader level of similar products.
Simple and fast: Simple operation steps, no need for additional instruments and special training, complete the test within 10 minutes.
Multiple specifications: 1 time, 5 times and 25 times in three packages, stored at room temperature, valid for up to 24 months.
Together with the Disposable sampling kit(swab sample), Disposable sampling kit(saliva sample), Automatic nucleic acid extractor, Nucleic acid extraction reagent, Novel coronavirus (SARS-CoV-2) nucleic acid detection kit (fluorescent PCR method), Novel coronavirus (SARS-CoV-2)/Influenza A/B virus nucleic acid detection kit ( fluorescent PCR method), Novel coronavirus (SARS-CoV-2) nucleic acid test kit (digital PCR method) and TD-1 digital PCR system, TargetingOne gains a total of eight novel coronavirus test-related reagents and instruments have been qualified for CE market access in the EU, providing three kinds of test methods: antigen, nucleic acid fluorescent PCR(qPCR) and nucleic acid digital PCR(dPCR), covering the entire process from sampling to nucleic acid and antigen detection.